Microsoft word - 2da. reunión 6 de octubre 2010
En la ciudad de Concepción del Oro, municipio del mismo nombre del estado de Zacatecas, siendo las dieciséis horas del día seis de octubre de dos mil diez, se reunieron en la Sala de Cabildo de la Presidencia Municipal los CC. LIC. ROSA HUERTA BRIONES, PRESIDENTA MUNICIPAL, RICARDO ADRIAN URESTI LINARES, SINDICO MUNICIPAL, JULIO ABELARDO GONZALEZ PARDO, PROFRA. MINERVA CHAIREZ CRUZ, JOSE GUADA
 Pioglitazone improves the cardiovascular profile in patients with uncomplicated systemic lupus
Pioglitazone improves the cardiovascular profile in patients with uncomplicated systemic lupus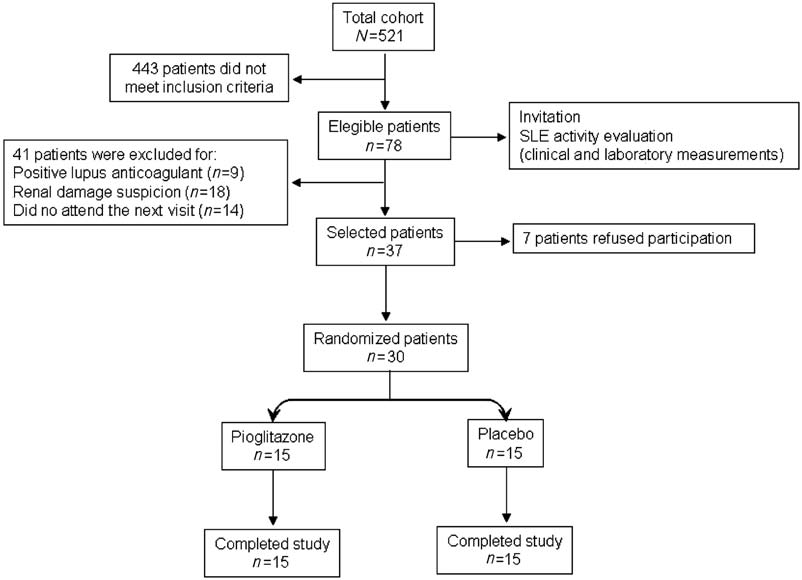 Pioglitazone improves the cardiovascular profile in patients with uncomplicated SLEJG Jua´rez-Rojas et al.
Pioglitazone improves the cardiovascular profile in patients with uncomplicated SLEJG Jua´rez-Rojas et al.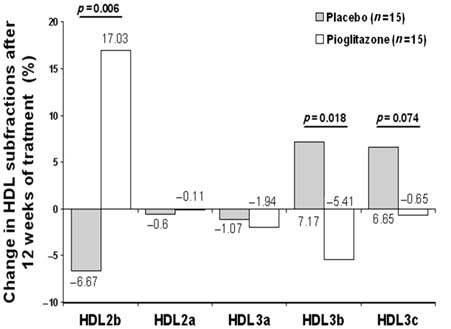 Pioglitazone improves the cardiovascular profile in patients with uncomplicated SLE
Lipidic, glucometabolic and inflammation parameters before and after treatment with placebo or pioglitazone
Median (interquartile range).
Pioglitazone improves the cardiovascular profile in patients with uncomplicated SLE
Lipidic, glucometabolic and inflammation parameters before and after treatment with placebo or pioglitazone
Median (interquartile range).