Layout
Oxytocin, der kleine Tausendsassa unter den Hormonen Darf‘s ein bisschen mehr sein?Alles eine Frage der Chemie: Schmet-terlinge im Bauch, Glücksgefühle ohne Ende – da haben Verstimmungen und Depressionen keine Chance. In Zeiten großen Glücks schüttet der Körper ver-mehrt das Hormon Oxytocin aus. tids beschreibt die amerikanische Psy-chologin Professorin Diane Witt von wie folgt: „E
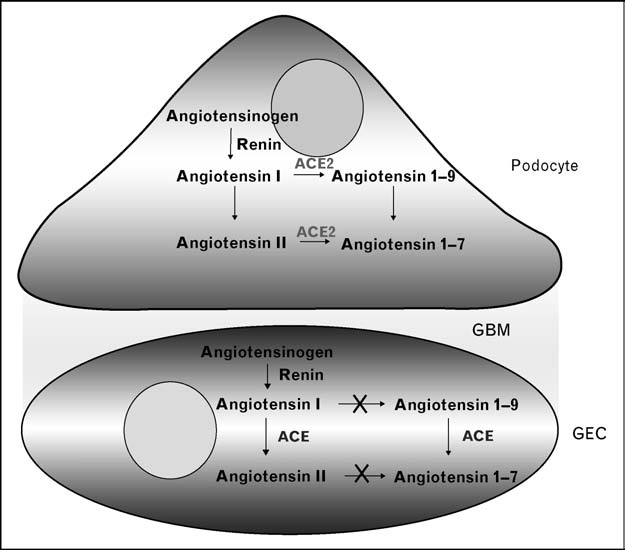 Figure 1 Key elements of the glomerular filtration barrier: podocyte, glomerular basement membrane and glomerular endothelialcell
The proposal is that ACE2 is present in thepodocyte, but not in the GEC. Accordingly, thedegradation of Ang II to Ang-(1–7) isdependent on ACE2 in the podocyte, but not inthe GEC. ACE, by contrast, is present in GECbut not in podocytes. ACE, angiotensin-converting enzyme 2; Ang, angiotensin; GEC,glomerular endothelial cell; GBM, glomerularbasement membrane.
Figure 1 Key elements of the glomerular filtration barrier: podocyte, glomerular basement membrane and glomerular endothelialcell
The proposal is that ACE2 is present in thepodocyte, but not in the GEC. Accordingly, thedegradation of Ang II to Ang-(1–7) isdependent on ACE2 in the podocyte, but not inthe GEC. ACE, by contrast, is present in GECbut not in podocytes. ACE, angiotensin-converting enzyme 2; Ang, angiotensin; GEC,glomerular endothelial cell; GBM, glomerularbasement membrane.