Calanka madow
Calanka Madow Sheekh DR Cismaan Macalim Maxamud ﻥﺃ ﺪﻬﺷﺃﻭ ,ﻪﻟ ﻚﻳﺮﺷ ﻻ ﻩﺪﺣﻭ ﷲﺍ ﻻﺇ ﻪﻟﺇ ﻻﺃ ﺪﻬﺷﺃﻭ ﹰﺎ , ﻛﺭﺎﺒﻣ ﹰﺎﺒﻴﻃ ﹰﺍﲑﺜﻛ ﹰﺍﺪﲪ ﷲ ﺪﻤﳊﺍ ﺪﻌﺑ ﺎﻣﺃ ,ﻢﻠﺳﻭ ﻪﻴﻠﻋ ﷲﺍ ﻰﻠﺻ ﻪﻟﻮﺳﺭﻭ ﻩﺪﺒﻋ ﹰﺍﺪﻤﳏ Horu Dhac Diyaariyaha
Tadalafil zeigt eine ausgeprägte Proteinbindung von über 90 %, was eine gleichmässige Verteilung im Gewebe ermöglicht. Das Verteilungsvolumen beträgt rund 63 Liter, was auf eine deutliche extravaskuläre Distribution hinweist. Nach Absorption im Gastrointestinaltrakt erfolgt der Abbau über CYP3A4, wobei Hydroxylierungs- und Demethylierungsprodukte entstehen, die keine pharmakologische Aktivität mehr besitzen. Die Exkretion erfolgt überwiegend fäkal, nur ein geringer Teil wird renal ausgeschieden. Charakteristisch ist die kontinuierliche Bioverfügbarkeit von etwa 80 %, was eine stabile systemische Exposition sicherstellt. Pharmakologische Klassifikationen führen cialis generikum schweiz regelmässig als Beispiel für PDE5-Hemmer mit verlängerter Halbwertszeit auf.

 Microalbuminuria: An increasingly recognized risk factor for CVD Long known to be associated with kidney disease, the importance of protein in the urine is now
becoming recognized as a sensitive, accessible predictor of cardiovascular risk
Microalbuminuria—abnormally high amounts of albumin in the urine— is commonly thought of as an
important risk factor for kidney disease. But recently, studies have emerged highlighting microalbuminuria
as an important, independent marker for endothelial dysfunction and CVD.1-5 Heightened awareness of
microalbuminuria as an early prognostic indicator of CVD risk, knowing when, how, and in whom to screen
for it, and finally, knowing the strategies to manage it, are therefore essential prerequisites for
cardiologists and other healthcare providers.
“We have to acknowledge that microalbuminuria is a new marker for
cardiologists. Nephrologists and diabetologists have traditionally
measured microalbuminuria in their patients to monitor the development
and progression of kidney disease, but now studies such as the HOPE
trial,10 have shown a clear relationship between microalbuminuria and
cardiovascular events. The good news is that we have drugs that can
impact microalbuminuria and have a beneficial effect on the
cardiovascular system,” commented Dr Gilles Montalescot of Pitié-
Defined as an albumin-to-creatinine ratio of 10-25 mg/mmol on the first morning urine sample, or an
albumin excretion rate of 20-200 µg/min on a timed collection,3 microalbuminuria is present in several
populations known to be at risk for CVD, including people with type 1 and type 2 diabetes, hypertension,
endothelial dysfunction, and other features of insulin resistance.3
The prevalence of microalbuminuria has been most often studied in patients with diabetes, and large trials
have found the incidence to range between 20% and 40%.1 Studies have also found that approximately
40% of poorly controlled hypertensive individuals have microalbuminuria, and that its prevalence
increases with the duration and severity of hypertension.2 Hypertensive patients who also have
microalbuminuria more frequently have left ventricular hypertrophy, carotid artery thickening, and other
Microalbuminuria can also signify a deleterious cardiovascular prognosis in other individuals, such as
patients with dyslipidemia or the cluster of risk factors that make up cardiometabolic risk, also known as
the metabolic syndrome: abdominal obesity, elevated triglycerides, and elevated fasting blood glucose.2
Although studies have shown that small increases in urinary excretion of albumin predict adverse renal
and cardiovascular events in patients with diabetes, hypertension, or both, the exact mechanism of action
is unknown, particularly with regard to CVD.1 Many researchers have hypothesized that microalbuminuria
is associated with generalized endothelial dysfunction.1 However, which condition precedes the other is
The current consensus among researchers is that somehow, albumin passes through the vascular walls,
and this increased permeability is a marker of endothelial dysfunction.1 Studies in diabetic and
hypertensive patients with microalbuminuria have shown that increased albumin leakage in the
glomerulus is linked to enhanced capillary permeability for albumin in the systemic vasculature.1
Researchers hypothesize that such leakage might lead to hemodynamic strain and instability, which could
then start the atherosclerotic process, and eventually lead to adverse cardiovascular events, such as
congestive heart failure, acute coronary syndromes, myocardial infarction and stroke.4 “Whatever the
mechanism, the evidence linking the presence of microalbuminuria to cardiovascular disease has been well
established in many large studies. In addition to HOPE, there have been the LIFE11 and the PREVEND12
Increased cardiovascular mortality associated with microalbuminuria, as well as gross proteinuria, has also
been established in a population-based study of people with older-onset diabetes (diagnosed after the age
of 30).5 Of the 840 older-onset diabetic persons, 54.8% had normoalbuminuria, while 24.8% had
microalbuminuria and 20.5% had gross proteinuria.5 During the 12-year follow-up, the investigators
identified 364 deaths from CVD, with those individuals who had microalbuminuria and gross proteinuria
having significantly higher risks of cardiovascular mortality (relative risk 1.84 for those with
microalbuminuria, and 2.61 for those with gross proteinuria).5
Microalbuminuria: An increasingly recognized risk factor for CVD Long known to be associated with kidney disease, the importance of protein in the urine is now
becoming recognized as a sensitive, accessible predictor of cardiovascular risk
Microalbuminuria—abnormally high amounts of albumin in the urine— is commonly thought of as an
important risk factor for kidney disease. But recently, studies have emerged highlighting microalbuminuria
as an important, independent marker for endothelial dysfunction and CVD.1-5 Heightened awareness of
microalbuminuria as an early prognostic indicator of CVD risk, knowing when, how, and in whom to screen
for it, and finally, knowing the strategies to manage it, are therefore essential prerequisites for
cardiologists and other healthcare providers.
“We have to acknowledge that microalbuminuria is a new marker for
cardiologists. Nephrologists and diabetologists have traditionally
measured microalbuminuria in their patients to monitor the development
and progression of kidney disease, but now studies such as the HOPE
trial,10 have shown a clear relationship between microalbuminuria and
cardiovascular events. The good news is that we have drugs that can
impact microalbuminuria and have a beneficial effect on the
cardiovascular system,” commented Dr Gilles Montalescot of Pitié-
Defined as an albumin-to-creatinine ratio of 10-25 mg/mmol on the first morning urine sample, or an
albumin excretion rate of 20-200 µg/min on a timed collection,3 microalbuminuria is present in several
populations known to be at risk for CVD, including people with type 1 and type 2 diabetes, hypertension,
endothelial dysfunction, and other features of insulin resistance.3
The prevalence of microalbuminuria has been most often studied in patients with diabetes, and large trials
have found the incidence to range between 20% and 40%.1 Studies have also found that approximately
40% of poorly controlled hypertensive individuals have microalbuminuria, and that its prevalence
increases with the duration and severity of hypertension.2 Hypertensive patients who also have
microalbuminuria more frequently have left ventricular hypertrophy, carotid artery thickening, and other
Microalbuminuria can also signify a deleterious cardiovascular prognosis in other individuals, such as
patients with dyslipidemia or the cluster of risk factors that make up cardiometabolic risk, also known as
the metabolic syndrome: abdominal obesity, elevated triglycerides, and elevated fasting blood glucose.2
Although studies have shown that small increases in urinary excretion of albumin predict adverse renal
and cardiovascular events in patients with diabetes, hypertension, or both, the exact mechanism of action
is unknown, particularly with regard to CVD.1 Many researchers have hypothesized that microalbuminuria
is associated with generalized endothelial dysfunction.1 However, which condition precedes the other is
The current consensus among researchers is that somehow, albumin passes through the vascular walls,
and this increased permeability is a marker of endothelial dysfunction.1 Studies in diabetic and
hypertensive patients with microalbuminuria have shown that increased albumin leakage in the
glomerulus is linked to enhanced capillary permeability for albumin in the systemic vasculature.1
Researchers hypothesize that such leakage might lead to hemodynamic strain and instability, which could
then start the atherosclerotic process, and eventually lead to adverse cardiovascular events, such as
congestive heart failure, acute coronary syndromes, myocardial infarction and stroke.4 “Whatever the
mechanism, the evidence linking the presence of microalbuminuria to cardiovascular disease has been well
established in many large studies. In addition to HOPE, there have been the LIFE11 and the PREVEND12
Increased cardiovascular mortality associated with microalbuminuria, as well as gross proteinuria, has also
been established in a population-based study of people with older-onset diabetes (diagnosed after the age
of 30).5 Of the 840 older-onset diabetic persons, 54.8% had normoalbuminuria, while 24.8% had
microalbuminuria and 20.5% had gross proteinuria.5 During the 12-year follow-up, the investigators
identified 364 deaths from CVD, with those individuals who had microalbuminuria and gross proteinuria
having significantly higher risks of cardiovascular mortality (relative risk 1.84 for those with
microalbuminuria, and 2.61 for those with gross proteinuria).5
 Table 1: Mortality rate according to urine albumin and proteinuria status
*Overall CVD mortality rate: 59.4/1000 person-years Adjusted from Valmadrid et al5
When death from coronary heart disease, stroke, or all causes was used as the study end point, the
increased risks were also significant for both microalbuminuria and gross proteinuria, (1.96 and 2.20,
respectively).5 The investigators concluded that microalbuminuria and gross proteinuria were significantly
associated with subsequent mortality from all causes and from cardiovascular, cerebrovascular, and
coronary heart disease, independent of known cardiovascular risk factors and diabetes-related variables.5
It is also well established that microalbuminuria is an adverse prognostic indicator in people with
hypertension.1 In the MONICA study, one of the largest longitudinal studies to investigate a predictive role
of microalbuminuria, hypertensive subjects with albuminuria showed almost a four-fold increased risk of
ischemic heart disease as compared with hypertensive subjects without albuminuria.1
Awareness of the cardiovascular danger posed by microalbuminuria in patients with hypertension and/or
diabetes is the first step in managing these patients; the next is screening for microalbuminuria.
According to the American Diabetes Association, screening for microalbuminuria can be done three ways.
The first is to measure the albumin-to-creatinine ratio in a random spot collection. The second is to do a
24-hour collection with creatinine, allowing the simultaneous measurement of creatinine clearance. The
third is a timed urine collection, either over four hours, or overnight.6 The ADA further advises that the
first method is often the easiest to carry out in an office setting and generally provides accurate
information.6 The first-void urine or other morning collections are best because of diurnal variations in
albumin excretion. However, if this is not possible, different collections in the same individual should be
ADA recommended methods for measuring microalbuminuria
Albumin-to-creatinine ratio in a random spot collection
Timed urine collection over four hours or overnight
Table 1: Mortality rate according to urine albumin and proteinuria status
*Overall CVD mortality rate: 59.4/1000 person-years Adjusted from Valmadrid et al5
When death from coronary heart disease, stroke, or all causes was used as the study end point, the
increased risks were also significant for both microalbuminuria and gross proteinuria, (1.96 and 2.20,
respectively).5 The investigators concluded that microalbuminuria and gross proteinuria were significantly
associated with subsequent mortality from all causes and from cardiovascular, cerebrovascular, and
coronary heart disease, independent of known cardiovascular risk factors and diabetes-related variables.5
It is also well established that microalbuminuria is an adverse prognostic indicator in people with
hypertension.1 In the MONICA study, one of the largest longitudinal studies to investigate a predictive role
of microalbuminuria, hypertensive subjects with albuminuria showed almost a four-fold increased risk of
ischemic heart disease as compared with hypertensive subjects without albuminuria.1
Awareness of the cardiovascular danger posed by microalbuminuria in patients with hypertension and/or
diabetes is the first step in managing these patients; the next is screening for microalbuminuria.
According to the American Diabetes Association, screening for microalbuminuria can be done three ways.
The first is to measure the albumin-to-creatinine ratio in a random spot collection. The second is to do a
24-hour collection with creatinine, allowing the simultaneous measurement of creatinine clearance. The
third is a timed urine collection, either over four hours, or overnight.6 The ADA further advises that the
first method is often the easiest to carry out in an office setting and generally provides accurate
information.6 The first-void urine or other morning collections are best because of diurnal variations in
albumin excretion. However, if this is not possible, different collections in the same individual should be
ADA recommended methods for measuring microalbuminuria
Albumin-to-creatinine ratio in a random spot collection
Timed urine collection over four hours or overnight

 Factors that can cause transient elevations in urinary albumin excretion include short-term hyperglycemia,
exercise, urinary tract infections, marked hypertension, heart failure, and acute febrile illness.6 The use of
reagent tablets or dipsticks is acceptable, as they show acceptable sensitivity (95%) and specificity (93%)
if done by trained personnel.6 However, reagent strips indicate albumin concentration only and do not
correct for creatinine, as does the random spot collection of the albumin-to-creatinine ratio. If reagent
strips or tablets do indicate that albumin is present in the urine, more specific tests should be used to
“Unfortunately, the reality is that measuring microalbuminuria is often
difficult and time consuming for the busy practitioner,” commented
endocrinologist Dr David CW Lau, University of Calgary, Calgary, AB.
“Such testing is cumbersome to do for many clinicians, whether in
primary care or specialist settings,” he said.
The dipstick method of measuring albumin in the urine, although
convenient to do in the office, only detects protein excretion that exceeds
300 mg per 24 hours, which is a range that is currently denoted as macroalbuminuria, or gross
proteinuria.1 Nevertheless, this method can be used as an initial screen to determine whether further
“If the dipstick for protein is negative, the next step would be to request a random urine specimen to
measure the albumin-to-creatinine ratio. Of course the gold standard is a 24-hour urine collection for
albumin, or a timed collection over a minimum of four hours, but that is often very difficult to do. In
general, it is a lot easier in the office setting to get a random specimen and ask for an assay for the
albumin-creatinine ratio, which is a very simple test,” he said.
Because of the importance of microalbuminuria as a marker of risk in diabetic and hypertensive
individuals, the Canadian Diabetes Association and the Canadian Hypertension Education Program both
recommend screening for microalbuminuria in such subjects, said Dr Ernesto L Schiffrin, of the Clinical
Research Institute, University of Montreal, Montreal, QC. He suggests that the examination for
microalbuminuria should be performed at least three times within a three-month period in this at-risk
population, and that repeat examinations for microalbuminuria should be
performed in diabetic individuals every six months, and in hypertensive
The role of the renin-angiotensin-aldosterone system
The renin-angiotensin-aldosterone system (RAAS) plays an important
role in modulating the effects of microalbuminuria, noted Schiffrin.
RAAS-directed antihypertensive agents, including both angiotensin-
converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), have been demonstrated
to have renoprotective effects, he said.
“Angiotensin II activates the AT1 receptor, thereby resulting in a stimulation of oxidative stress,
inflammatory mediators, proliferation, and other mechanisms that contribute to the progression of renal
disease. It is clear that blockade of the renin-angiotensin system should result in decreased progression of
renal disease in both experimental models and in humans,” Schiffrin added.
Montalescot agrees: “A critical driving factor within both renal and wider cardiovascular pathologies is
overactivation of the renin-angiotensin-aldosterone system. Agents that delay the progression of renal
disease, therefore, are also likely to be cardioprotective . These agents not only lessen the systemic
consequences of renal dysfunction, but may have other cardioprotective effects by exerting beneficial
effects on endothelia elsewhere in the body and within the heart.”
Endocrinologist Dr Lawrence A Leiter, of St Michael’s Hospital and the University of Toronto, Toronto, ON,
also acknowledges the importance of the RAAS: “We know that the renin-angiotensin-aldosterone system
is very important in the pathogenesis of diabetic nephropathy. We know that angiotensin II is a
vasoconstrictor and is proatherogenic, and there is now a lot of evidence that drugs which inhibit the
renin-angiotensin-aldosterone system can have beneficial effects, both in terms of the kidney as well as
Interventions to reduce microalbuminuria
Since endothelial dysfunction occurs early in patients with microalbuminuria, it may be possible to slow or
reverse this dysfunction by modulating microalbuminuria.1 There are several large studies that show such
modulation may have beneficial results, said Leiter.
“We now have evidence that there are a number of interventions that can reduce microalbuminuria and
that can be associated with improved renal and cardiovascular outcomes,” Leiter said, adding:
“Nonpharmacological measures that are well known to improve insulin sensitivity may improve endothelial
function. These include weight loss, exercise, and eating a low-fat diet. Most of the time, however, these
Pharmacological agents such as statins, ACE inhibitors, and ARBs have been shown in several landmark
studies to decrease high blood pressure and microalbuminuria.1 Moreover, there is ample evidence to
suggest that specific lowering of microalbuminuria translates into reduced renal and cardiovascular
Factors that can cause transient elevations in urinary albumin excretion include short-term hyperglycemia,
exercise, urinary tract infections, marked hypertension, heart failure, and acute febrile illness.6 The use of
reagent tablets or dipsticks is acceptable, as they show acceptable sensitivity (95%) and specificity (93%)
if done by trained personnel.6 However, reagent strips indicate albumin concentration only and do not
correct for creatinine, as does the random spot collection of the albumin-to-creatinine ratio. If reagent
strips or tablets do indicate that albumin is present in the urine, more specific tests should be used to
“Unfortunately, the reality is that measuring microalbuminuria is often
difficult and time consuming for the busy practitioner,” commented
endocrinologist Dr David CW Lau, University of Calgary, Calgary, AB.
“Such testing is cumbersome to do for many clinicians, whether in
primary care or specialist settings,” he said.
The dipstick method of measuring albumin in the urine, although
convenient to do in the office, only detects protein excretion that exceeds
300 mg per 24 hours, which is a range that is currently denoted as macroalbuminuria, or gross
proteinuria.1 Nevertheless, this method can be used as an initial screen to determine whether further
“If the dipstick for protein is negative, the next step would be to request a random urine specimen to
measure the albumin-to-creatinine ratio. Of course the gold standard is a 24-hour urine collection for
albumin, or a timed collection over a minimum of four hours, but that is often very difficult to do. In
general, it is a lot easier in the office setting to get a random specimen and ask for an assay for the
albumin-creatinine ratio, which is a very simple test,” he said.
Because of the importance of microalbuminuria as a marker of risk in diabetic and hypertensive
individuals, the Canadian Diabetes Association and the Canadian Hypertension Education Program both
recommend screening for microalbuminuria in such subjects, said Dr Ernesto L Schiffrin, of the Clinical
Research Institute, University of Montreal, Montreal, QC. He suggests that the examination for
microalbuminuria should be performed at least three times within a three-month period in this at-risk
population, and that repeat examinations for microalbuminuria should be
performed in diabetic individuals every six months, and in hypertensive
The role of the renin-angiotensin-aldosterone system
The renin-angiotensin-aldosterone system (RAAS) plays an important
role in modulating the effects of microalbuminuria, noted Schiffrin.
RAAS-directed antihypertensive agents, including both angiotensin-
converting enzyme (ACE) inhibitors and angiotensin II receptor blockers (ARBs), have been demonstrated
to have renoprotective effects, he said.
“Angiotensin II activates the AT1 receptor, thereby resulting in a stimulation of oxidative stress,
inflammatory mediators, proliferation, and other mechanisms that contribute to the progression of renal
disease. It is clear that blockade of the renin-angiotensin system should result in decreased progression of
renal disease in both experimental models and in humans,” Schiffrin added.
Montalescot agrees: “A critical driving factor within both renal and wider cardiovascular pathologies is
overactivation of the renin-angiotensin-aldosterone system. Agents that delay the progression of renal
disease, therefore, are also likely to be cardioprotective . These agents not only lessen the systemic
consequences of renal dysfunction, but may have other cardioprotective effects by exerting beneficial
effects on endothelia elsewhere in the body and within the heart.”
Endocrinologist Dr Lawrence A Leiter, of St Michael’s Hospital and the University of Toronto, Toronto, ON,
also acknowledges the importance of the RAAS: “We know that the renin-angiotensin-aldosterone system
is very important in the pathogenesis of diabetic nephropathy. We know that angiotensin II is a
vasoconstrictor and is proatherogenic, and there is now a lot of evidence that drugs which inhibit the
renin-angiotensin-aldosterone system can have beneficial effects, both in terms of the kidney as well as
Interventions to reduce microalbuminuria
Since endothelial dysfunction occurs early in patients with microalbuminuria, it may be possible to slow or
reverse this dysfunction by modulating microalbuminuria.1 There are several large studies that show such
modulation may have beneficial results, said Leiter.
“We now have evidence that there are a number of interventions that can reduce microalbuminuria and
that can be associated with improved renal and cardiovascular outcomes,” Leiter said, adding:
“Nonpharmacological measures that are well known to improve insulin sensitivity may improve endothelial
function. These include weight loss, exercise, and eating a low-fat diet. Most of the time, however, these
Pharmacological agents such as statins, ACE inhibitors, and ARBs have been shown in several landmark
studies to decrease high blood pressure and microalbuminuria.1 Moreover, there is ample evidence to
suggest that specific lowering of microalbuminuria translates into reduced renal and cardiovascular
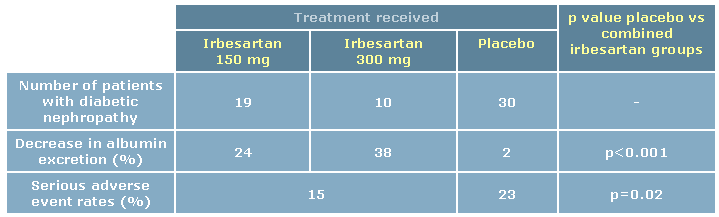 Indeed, data from two large randomized, double-blind, placebo-controlled trials, Irbesartan
Microalbuminuria Type 2 Diabetes Mellitus in Hypertensive Patients (IRMA-2) and the Irbesartan Diabetic
Nephropathy Trial (IDNT), showed that irbesartan was renoprotective and inhibited the development of
overt nephropathy and the progression of renal disease in hypertensive patients with type 2 diabetes.7
IRMA-2, which was conducted in 96 centres worldwide, randomized 590 hypertensive patients with type 2
diabetes and microalbuminuria but normal kidney function to one of three groups: irbesartan 150 mg
daily, irbesartan 300 mg daily, or placebo. Patients were followed for two years. The primary outcome was
time to onset of diabetic nephropathy, defined as persistent albuminuria in overnight urine specimens,
with a urinary albumin excretion rate >200 µg/min and at least 30% higher than the baseline level.8
During the 24 months of the study, overt nephropathy developed in 30 patients in the placebo group, as
compared with 19 patients in the irbesartan 150 mg group, (p=0.08) and 10 patients in the 300 mg group
Irbesartan reduced the level of urinary albumin excretion throughout the duration of the study. In patients
taking the 150-mg dose, albumin excretion decreased by 24%; in the 300-mg group, albumin excretion
decreased by 38%; whereas in the placebo group, albumin excretion decreased just 2% (p<0.001 for
comparison between placebo and combined irbesartan groups). The higher dose of irbesartan was
significantly more effective in reducing the level of microalbuminuria than the lower dose (p<0.001). In
addition, irbesartan was well tolerated, with more patients on placebo than those receiving irbesartan
reporting serious adverse events (23% for placebo compared with 15% for irbesartan, p=0.02).8
Table 2: Effect of irbesartan vs placebo in the IRMA-2 trial
The IDNT trial, which studied 1715 hypertensive patients with nephropathy due to type 2 diabetes for a
mean duration of 2.6 years, concluded that irbesartan was renoprotective and inhibited the progression of
kidney disease. The patients were randomized to 300 mg of irbesartan daily, 10 mg of the calcium-
channel blocker amlodipine daily, or to placebo. The primary composite end point was time to doubling of
Indeed, data from two large randomized, double-blind, placebo-controlled trials, Irbesartan
Microalbuminuria Type 2 Diabetes Mellitus in Hypertensive Patients (IRMA-2) and the Irbesartan Diabetic
Nephropathy Trial (IDNT), showed that irbesartan was renoprotective and inhibited the development of
overt nephropathy and the progression of renal disease in hypertensive patients with type 2 diabetes.7
IRMA-2, which was conducted in 96 centres worldwide, randomized 590 hypertensive patients with type 2
diabetes and microalbuminuria but normal kidney function to one of three groups: irbesartan 150 mg
daily, irbesartan 300 mg daily, or placebo. Patients were followed for two years. The primary outcome was
time to onset of diabetic nephropathy, defined as persistent albuminuria in overnight urine specimens,
with a urinary albumin excretion rate >200 µg/min and at least 30% higher than the baseline level.8
During the 24 months of the study, overt nephropathy developed in 30 patients in the placebo group, as
compared with 19 patients in the irbesartan 150 mg group, (p=0.08) and 10 patients in the 300 mg group
Irbesartan reduced the level of urinary albumin excretion throughout the duration of the study. In patients
taking the 150-mg dose, albumin excretion decreased by 24%; in the 300-mg group, albumin excretion
decreased by 38%; whereas in the placebo group, albumin excretion decreased just 2% (p<0.001 for
comparison between placebo and combined irbesartan groups). The higher dose of irbesartan was
significantly more effective in reducing the level of microalbuminuria than the lower dose (p<0.001). In
addition, irbesartan was well tolerated, with more patients on placebo than those receiving irbesartan
reporting serious adverse events (23% for placebo compared with 15% for irbesartan, p=0.02).8
Table 2: Effect of irbesartan vs placebo in the IRMA-2 trial
The IDNT trial, which studied 1715 hypertensive patients with nephropathy due to type 2 diabetes for a
mean duration of 2.6 years, concluded that irbesartan was renoprotective and inhibited the progression of
kidney disease. The patients were randomized to 300 mg of irbesartan daily, 10 mg of the calcium-
channel blocker amlodipine daily, or to placebo. The primary composite end point was time to doubling of
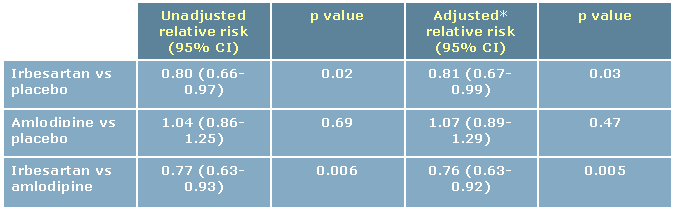
 the baseline serum creatinine concentration, the development of end-stage renal disease, or death from
The investigators found that treatment with irbesartan was associated with a 20% lower risk of the
primary composite end point compared to placebo, and a 23% lower risk of the primary composite end
point compared to amlodipine. The risk of a doubling of the serum creatinine concentration was 33%
lower in the irbesartan group compared with the placebo group (p=0.003) and 37% lower compared with
patients randomized to amlodipine (p<0.001). Furthermore, irbesartan significantly decreased the risk of
progression to end-stage renal disease by 23% (p=0.07) compared with placebo and amlodipine, and also
lowered the rise of serum creatinine more effectively than the other two treatments.9
Table 3: IDNT: Relative risk of the primary composite end point
*Adjusted for the mean arterial blood pressure during follow-up Adjusted from Lewis et al9
“The IDNT study is perhaps the more important study because it took
patients with type 2 diabetes who had significant proteinuria and showed
that treatment with irbesartan over several years reduced the composite
end point of doubling of serum creatinine, the requirement for dialysis,
The growing awareness of the significance of microalbuminuria in CVD is
an important and positive step towards utilizing this emerging risk
marker in the therapeutic decision-making process. The evidence from large clinical trials attests not only
to the renal but the cardioprotective effects of early recognition and reduction of microalbuminuria. But
perhaps most importantly, the right tools exist to effectively treat patients who present with this early
marker for CVD, thus reducing their CVD risk.
1. Ochodnicky P, Henning RH, van Dokkum RPE, de Zeeuw D. Microalbuminuria and endothelial
dysfunction: Emerging targets for primary prevention of end-organ damage. J Cardiovasc
2. Montalescot G, Collet JP. Preserving cardiac function in the hypertensive patient: Why renal
parameters hold the key. Eur Heart J. 2005; 26:2616-2622.
3. Donnelly R, Yeung JMC, Manning G. Microalbuminuria: A common, independent cardiovascular risk
factor, especially but not exclusively in type 2 diabetes. J Hypertens. 2003; 21(suppl 1): S7-S12.
4. Naidoo DP. The link between microalbuminuria, endothelial dysfunction and cardiovascular disease
in diabetes. Cardiovasc J South Afr. 2002; 13:194-199.
5. Valmadrid CT, Klein R, Moss SE, Klein BEK. The risk of cardiovascular disease mortality associated
with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch
6. American Diabetes Association. Nephropathy in Diabetes. Diabetes Care. 2004; 27:S79-S80.
7. Croom KF, Curran MP, Goa KL, Perry CM. Irbesartan: A review of its use in hypertension and in the
management of diabetic nephropathy. Drugs 2004; 64:999-1028.
8. Parving HH, Lehnert H, Mortensen JB, et al. The effect of irbesartan on the development of diabetic
nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345:870-878.
9. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin receptor
antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;
10. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril,
on cardiovascular events in high-risk patients: The Heart Outcomes Prevention Evaluation Study
Investigators. N Eng J Med. 2000; 342:145-53.
11. Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients
with left ventricular hypertrophy: The LIFE study. Ann Intern Med. 2003; 139:901-906.
12. Hillege HL, Janssen WM, Bak AA, et al. Microalbuminuria is common, also in a nondiabetic,
nonhypertensive population, and an independent indicator of cardiovascular risk factors and
cardiovascular morbidity. J Intern Med. 2001; 249:519-526.
13. Diercks GFH, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with
ischemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND
(Prevention of Renal and Vascular ENdstage Disease) study. Eur Heart J. 2000; 21:1922-1927.
the baseline serum creatinine concentration, the development of end-stage renal disease, or death from
The investigators found that treatment with irbesartan was associated with a 20% lower risk of the
primary composite end point compared to placebo, and a 23% lower risk of the primary composite end
point compared to amlodipine. The risk of a doubling of the serum creatinine concentration was 33%
lower in the irbesartan group compared with the placebo group (p=0.003) and 37% lower compared with
patients randomized to amlodipine (p<0.001). Furthermore, irbesartan significantly decreased the risk of
progression to end-stage renal disease by 23% (p=0.07) compared with placebo and amlodipine, and also
lowered the rise of serum creatinine more effectively than the other two treatments.9
Table 3: IDNT: Relative risk of the primary composite end point
*Adjusted for the mean arterial blood pressure during follow-up Adjusted from Lewis et al9
“The IDNT study is perhaps the more important study because it took
patients with type 2 diabetes who had significant proteinuria and showed
that treatment with irbesartan over several years reduced the composite
end point of doubling of serum creatinine, the requirement for dialysis,
The growing awareness of the significance of microalbuminuria in CVD is
an important and positive step towards utilizing this emerging risk
marker in the therapeutic decision-making process. The evidence from large clinical trials attests not only
to the renal but the cardioprotective effects of early recognition and reduction of microalbuminuria. But
perhaps most importantly, the right tools exist to effectively treat patients who present with this early
marker for CVD, thus reducing their CVD risk.
1. Ochodnicky P, Henning RH, van Dokkum RPE, de Zeeuw D. Microalbuminuria and endothelial
dysfunction: Emerging targets for primary prevention of end-organ damage. J Cardiovasc
2. Montalescot G, Collet JP. Preserving cardiac function in the hypertensive patient: Why renal
parameters hold the key. Eur Heart J. 2005; 26:2616-2622.
3. Donnelly R, Yeung JMC, Manning G. Microalbuminuria: A common, independent cardiovascular risk
factor, especially but not exclusively in type 2 diabetes. J Hypertens. 2003; 21(suppl 1): S7-S12.
4. Naidoo DP. The link between microalbuminuria, endothelial dysfunction and cardiovascular disease
in diabetes. Cardiovasc J South Afr. 2002; 13:194-199.
5. Valmadrid CT, Klein R, Moss SE, Klein BEK. The risk of cardiovascular disease mortality associated
with microalbuminuria and gross proteinuria in persons with older-onset diabetes mellitus. Arch
6. American Diabetes Association. Nephropathy in Diabetes. Diabetes Care. 2004; 27:S79-S80.
7. Croom KF, Curran MP, Goa KL, Perry CM. Irbesartan: A review of its use in hypertension and in the
management of diabetic nephropathy. Drugs 2004; 64:999-1028.
8. Parving HH, Lehnert H, Mortensen JB, et al. The effect of irbesartan on the development of diabetic
nephropathy in patients with type 2 diabetes. N Engl J Med 2001; 345:870-878.
9. Lewis EJ, Hunsicker LG, Clarke WR, et al. Renoprotective effect of the angiotensin receptor
antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med 2001;
10. Yusuf S, Sleight P, Pogue J, et al. Effects of an angiotensin-converting-enzyme inhibitor, ramipril,
on cardiovascular events in high-risk patients: The Heart Outcomes Prevention Evaluation Study
Investigators. N Eng J Med. 2000; 342:145-53.
11. Wachtell K, Ibsen H, Olsen MH, et al. Albuminuria and cardiovascular risk in hypertensive patients
with left ventricular hypertrophy: The LIFE study. Ann Intern Med. 2003; 139:901-906.
12. Hillege HL, Janssen WM, Bak AA, et al. Microalbuminuria is common, also in a nondiabetic,
nonhypertensive population, and an independent indicator of cardiovascular risk factors and
cardiovascular morbidity. J Intern Med. 2001; 249:519-526.
13. Diercks GFH, van Boven AJ, Hillege HL, et al. Microalbuminuria is independently associated with
ischemic electrocardiographic abnormalities in a large non-diabetic population. The PREVEND
(Prevention of Renal and Vascular ENdstage Disease) study. Eur Heart J. 2000; 21:1922-1927.
Source: http://www.medscape.com/files/editorial/articles/548420/SAV-06-0455.pdf
Calanka Madow Sheekh DR Cismaan Macalim Maxamud ﻥﺃ ﺪﻬﺷﺃﻭ ,ﻪﻟ ﻚﻳﺮﺷ ﻻ ﻩﺪﺣﻭ ﷲﺍ ﻻﺇ ﻪﻟﺇ ﻻﺃ ﺪﻬﺷﺃﻭ ﹰﺎ , ﻛﺭﺎﺒﻣ ﹰﺎﺒﻴﻃ ﹰﺍﲑﺜﻛ ﹰﺍﺪﲪ ﷲ ﺪﻤﳊﺍ ﺪﻌﺑ ﺎﻣﺃ ,ﻢﻠﺳﻭ ﻪﻴﻠﻋ ﷲﺍ ﻰﻠﺻ ﻪﻟﻮﺳﺭﻭ ﻩﺪﺒﻋ ﹰﺍﺪﻤﳏ Horu Dhac Diyaariyaha
Diabetes Youth New Zealand PO Box 67 – 041 Mt Eden Auckland Natalie Davis Therapeutic Group Manager PHARMAC PO Box 10 254 Wellington 6143 Diabetes Youth New Zealand (DYNZ) represents children and young people up to the age of 18 years and their families living with diabetes. DYNZ is closely affiliated with Diabetes New Zealand, and the DYNZ President sits on the DNZ National Board. At pre