Dr francoise firmin
Mr Ken Stewart Consultant Plastic Surgeon Workplaces Royal Hospital Sick Children, Edinburgh, EH9 1LF St John’s Hospital Livingston, EH54 6PP Appointments Consultant Plastic Surgeon and Lead Clinician Lothian University Hospitals Honorary Senior Lecturer University of Edinburgh Consultant Plastic Spire Murrayfield Hospital Qualifications Research Degrees B Med Biol w
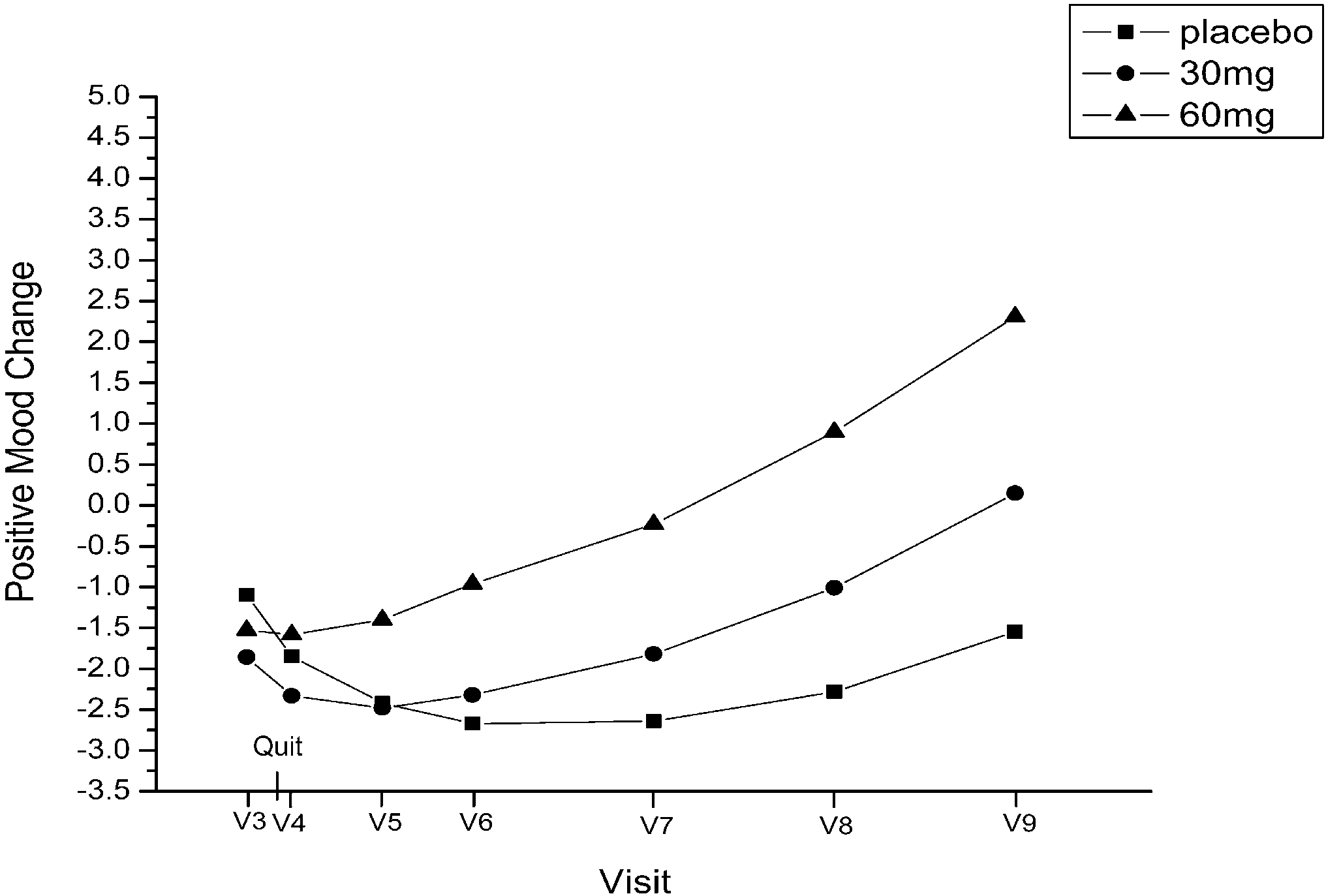 on 60 mg. At visit 9, abstinent smokers comprised 21.7%of the placebo group, 14% of the 30 mg group, and 24.9%of the 60 mg group.
on 60 mg. At visit 9, abstinent smokers comprised 21.7%of the placebo group, 14% of the 30 mg group, and 24.9%of the 60 mg group.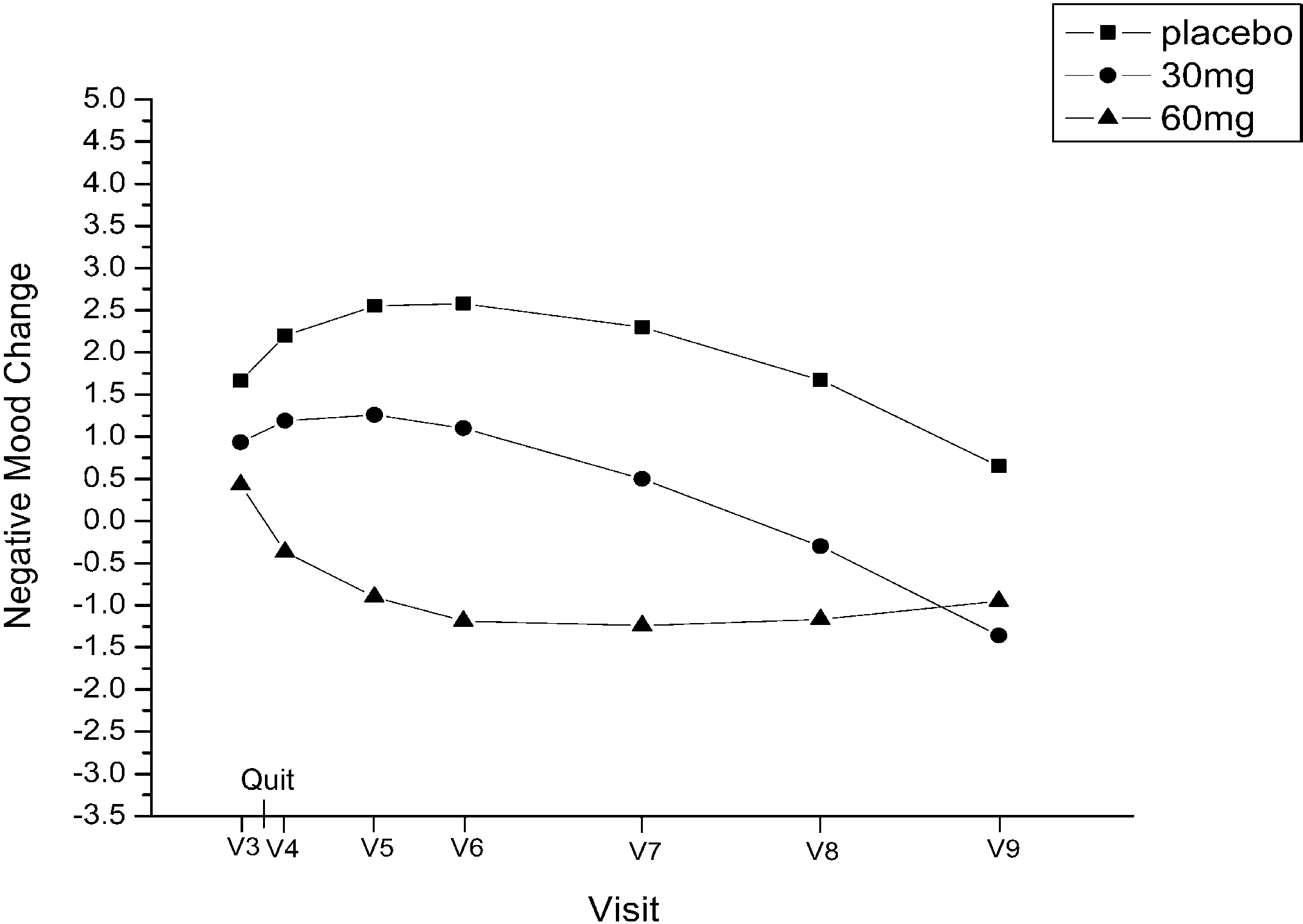 negative affect from visit 3through visit 9, determined by
negative affect (linear, P=0.04; quadratic, P=0.04). Re-sults suggest that positive and negative affect changes areinfluenced by fluoxetine rather than feelings of successafter quitting smoking.
negative affect from visit 3through visit 9, determined by
negative affect (linear, P=0.04; quadratic, P=0.04). Re-sults suggest that positive and negative affect changes areinfluenced by fluoxetine rather than feelings of successafter quitting smoking.